- Joined
- Nov 3, 2022
- Messages
- 3
- Reaction score
- 0
- Points
- 1
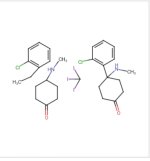
Ok, so I need an alternative K synth where I can attach the Oxo at the 4 position rather than the 2.
The only synth that seems remotely able to do is the new Iranian synth but swapping out the Oxophenyl for a 1,4-dioxo-phenyl ring and then cleaving the oxo with a deoxidizing agent after protecting the 4-oxo with .... (What would work best here and how do I selectively protect just the 4-oxo rather than the 1?)??
If anyone could give me any tips I'd be very grateful. I assume after the deoxidation I just follow the rest of the synth to the letter, right?
The only synth that seems remotely able to do is the new Iranian synth but swapping out the Oxophenyl for a 1,4-dioxo-phenyl ring and then cleaving the oxo with a deoxidizing agent after protecting the 4-oxo with .... (What would work best here and how do I selectively protect just the 4-oxo rather than the 1?)??
If anyone could give me any tips I'd be very grateful. I assume after the deoxidation I just follow the rest of the synth to the letter, right?