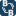Given the traditional Henry reaction, and given this ratio: Benzaldehyde 1000 ml,
nitroethane 1000 ml, glacial acetic acid 250 ml and n-butylamine 50 ml
Explanation:
- Benzaldehyde (C6H5CHOC6H5CHO):
- This aromatic compound provides the benzene ring and an aldehyde group.
- Nitroethane (CH3−CH2−NO2CH3−CH2−NO2):
- Contributes the nitro group (-NO2).
- Acetic Acid (CH3−COOHCH3−COOH):
- Acts as a weak acid catalyst and provides an acetyl group.
- n-Butylamine (C4H9NH2C4H9NH2):
- Functions as a base in the reaction.
- Product (Phenyl-2-nitropropene):
- The main product formed is Phenyl-2-nitropropene.
Let's break down the reaction for the formation of Phenyl-2-nitropropene from the given reactants:
Step 1: Nucleophilic Addition of Nitroethane to Benzaldehyde
- Formation of the Enamine Intermediate:
- n-Butylamine (C4H9NH2C4H9NH2) reacts with benzaldehyde (C6H5CHOC6H5CHO) to form an enamine intermediate.
- The lone pair of electrons on the nitrogen atom of n-butylamine attacks the carbonyl carbon of benzaldehyde, resulting in the formation of the enamine.
\ceC6H5CHO+C4H9NH2−>C6H5CH=CH−NH−C4H9\ceC6H5CHO+C4H9NH2−>C6H5CH=CH−NH−C4H9
Step 2: Nitroethane Addition to the Enamine
- Nitroethane Addition:
- Nitroethane (CH3−CH2−NO2CH3−CH2−NO2) adds to the enamine intermediate through a nucleophilic addition reaction.
- The nitronate ion attacks the enamine, forming a new carbon-carbon bond and generating the nitroalkane intermediate.
\ceC6H5CH=CH−NH−C4H9+CH3−CH2−NO2−>C6H5CH=CH−NO2+C4H9NH2\ceC6H5CH=CH−NH−C4H9+CH3−CH2−NO2−>C6H5CH=CH−NO2+C4H9NH2
Step 3: Formation of Phenyl-2-nitropropene
- Dehydration and Formation of Phenyl-2-nitropropene:
- Acetic acid (CH3−COOHCH3−COOH) reacts with the nitroalkane intermediate, leading to the elimination of water (dehydration) and forming Phenyl-2-nitropropene.
\ceC6H5CH=CH−NO2+CH3−COOH−>C6H5CH=CH−NO2+H2O\ceC6H5CH=CH−NO2+CH3−COOH−>C6H5CH=CH−NO2+H2O
Overall Reaction: \ceBenzaldehyde+Nitroethane+AceticAcid+n−Butylamine−>Phenyl−2−nitropropene+OtherProducts−>\ceBenzaldehyde+Nitroethane+AceticAcid+n−Butylamine−>Phenyl−2−nitropropene+OtherProducts
Key Points:
- The reaction involves multiple steps, starting with the formation of an enamine intermediate.
- Nucleophilic addition and dehydration reactions contribute to the synthesis of Phenyl-2-nitropropene.
- Reaction conditions, such as temperature and concentrations, influence the outcome, and optimization may be required for optimal yields
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Given the variant of the Henry reaction, and given this ratio: 80ml benzaldehyde 80ml nitroethane 60g anhydrous ammonium acetate 70ml GAA
I wont rewrite the reaction mechanism as we should assume it is the same?
Substituting n-butylamine with ammonium acetate in the reaction you provided would significantly alter the reaction pathway. Ammonium acetate typically acts as a catalyst in some reactions but does not serve the same nucleophilic function as n-butylamine. The reaction would likely follow a different mechanism, and the final product could differ.
Here's a brief overview of the expected changes:
- Formation of the Enamine Intermediate:
- Instead of a nucleophilic attack by the lone pair of nitrogen in n-butylamine, ammonium acetate may facilitate the reaction differently. The reaction mechanism would depend on the specific conditions.
- Nitroethane Addition to the Enamine:
- The addition of nitroethane to the enamine intermediate might still occur, but the details of the reaction would be influenced by the nature of the intermediate formed with ammonium acetate.
- Formation of Phenyl-2-nitropropene:
- The dehydration step involving acetic acid may still occur, but the specifics could vary, and the overall outcome might produce different by-products or isomers.
Balancing the reaction with ammonium acetate would require a detailed understanding of the specific reaction conditions, concentrations, and mechanism, which may not be readily available without experimental data. i will run the numbers again after posting this in case i am wrong.
In summary, while ammonium acetate can play a role in some reactions, substituting it for n-butylamine in the given context would likely result in a different reaction pathway and possibly a different final product. Detailed experimental investigation and optimization would be needed to determine the specific outcome.
Lets further investigate and see if you can find any reason it should work?
This is the values for n-Butylamine
This is the values for Ammonium Acetate
n-butylamine might not work or could lead to different outcomes:
- Difference in Reactivity:
- n-Butylamine is a primary amine with a nucleophilic amino group, allowing it to participate in nucleophilic addition reactions. Ammonium acetate, being a salt, lacks a nucleophilic amino group. Its reaction behavior may involve proton transfer or catalytic activity rather than direct nucleophilic attacks.
- Role of Ammonium Acetate:
- Ammonium acetate is often used as a catalyst or for facilitating certain reactions through its acidic or basic properties. However, its specific role depends on the reaction conditions and the nature of the reactants. In the provided reaction, the absence of a nucleophilic group in ammonium acetate may limit its direct involvement in certain steps.
- Reaction Mechanism:
- The substitution of n-butylamine with ammonium acetate could alter the overall reaction mechanism. The nucleophilic attack, which is crucial in the formation of the enamine intermediate, might not proceed as expected with ammonium acetate.
- By-Products and Side Reactions:
- Different reaction pathways may lead to the formation of alternative by-products or side reactions. The selectivity and efficiency of the reaction may be influenced by the specific reagents and conditions.
- Optimization Challenges:
- Even if a reaction involving ammonium acetate were possible, achieving optimal yields and selectivity might require extensive optimization of reaction conditions such as temperature, concentration, and reaction time.
- Balancing the Equation:
- Substituting ammonium acetate might also necessitate adjustments to the reaction stoichiometry to balance the equation. This depends on the specific reactions that ammonium acetate undergoes under the given conditions.
In essence, the choice of a reaction partner is critical in organic synthesis, and each reagent contributes distinct functional groups and reactivity patterns. Without experimental data or specific details about the reaction conditions, it's challenging to predict the exact outcome of substituting ammonium acetate for n-butylamine in the given reaction. Experimental testing would be required to explore the viability of this substitution and understand the resulting chemistry.
See attatchments and see if you can make sence of it. i cant. the numbers dont add up the differens in buffur solution, and what is it acting as a catalyst for? dont get me wrong, i accualy hope i am wrong here, because it is way cheaper and easyer to make Ammonium Acetate, then n-butylamine, thats for sure.