- Language
- 🇺🇸
- Joined
- Oct 17, 2023
- Messages
- 87
- Reaction score
- 96
- Points
- 18
- Deals
- 20
SYNTHESIS:
A solution of 100 g of 2,5-dimethoxybenzaldehyde in 220 g nitromethane was treated with 10 g anhydrous ammonium acetate, and heated on a steam bath for 2.5 h with occasional swirling. The deep-red reaction mixture was stripped of the excess nitromethane under vacuum, and the residue crystallized spontaneously. This crude nitrostyrene was purified by grinding under IPA, filtering, and air-drying, to yield 85 g of 2,5-dimethoxy-beta-nitrostyrene as a yellow-orange product of adequate purity for the next step. Further purification can be achieved by recrystallization from boiling IPA.
In a round-bottomed 2 L flask equipped with a magnetic stirrer and placed under an inert atmosphere, there was added 750 mL anhydrous THF, containing 30 g LAH. There was then added, in THF solution, 60 g 2,5-dimethoxy-beta-nitrostyrene. The final solution was a dirty yellow-brown color, and it was kept at reflux temperature for 24 h. After cooling, the excess hydride was destroyed by the dropwise addition of IPA. Then 30 mL 15% NaOH was added to convert the inorganic solids to a filterable mass. The reaction mixture was filtered and the filter cake washed first with THF and then with MeOH. The combined mother liquors and washings
were freed of solvent under vacuum and the residue suspended in 1.5 L H2O. This was acidified with HCl, washed with with 3x100 mL CH2Cl2, made strongly basic with 25% NaOH, and reextracted with 4x100 mL CH2Cl2. The pooled extracts were stripped of solvent under vacuum, yielding 26 g of oily residue, which was distilled at 120-130 °C at 0.5 mm/Hg to give 21g of a white oil, 2,5-dimethoxy-phenethylamine (2C-H) which picks up carbon dioxide from the air very quickly.
To a well-stirred solution of 24.8 g 2,5-dimethoxyphenethylamine in 40 mL glacial acetic acid, there was added 22 g elemental bromine dissolved in 40 mL acetic acid. After a couple of min, there was the formation of solids and the simultaneous evolution of considerable heat. The reaction mixture was allowed to return to room temperature, filtered, and the solids washed sparingly with cold acetic acid. This was the hydrobromide salt. There are many complicated salt forms, both polymorphs and hydrates, that can make the isolation and characterization of 2C-B treacherous. The happiest route is to form the insoluble hydrochloride salt by way of the free base. The entire mass of acetic acid-wet salt was dissolved in warm H2O, made basic to at least pH 11 with 25% NaOH, and extracted with 3x100 mL CH2Cl2. Removal of the solvent gave 33.7 g of residue which was distilled at 115-130 °C at 0.4 mm/Hg.
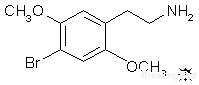
The white oil, 27.6 g, was dissolved in 50 mL H2O containing 7.0 g acetic acid. This clear solution was vigorous stirred, and treated with 20 mL concentrated HCl. There was an immediate formation of the anhydrous salt of 2,5-dimethoxy-4-bromophenethylamine hydrochloride (2C-B). This mass of crystals was removed by filtration (it can be loosened considerably by the addition of another 60 mL H2O), washed with a little H2O, and then with several 50 mL portions of Et2O. When completely air-dry, there was obtained 31.05 g of fine white needles, with a mp of 237-239 °C with decomposition. When there is too much H2O present at the time of adding the final concentrated HCl, a hydrated form of 2C-B is obtained. The hydrobromide salt melts at 214.5- 215 °C. The acetate salt was reported to have a mp of 208-209 °C.
DOSAGE: 12 - 24 mg.
DURATION: 4 - 8 h.
>> If someone tried to make this synthesis and can come up with pictures and more details would be nice, or even a video if I don't ask too much, I think many would be interested.
A solution of 100 g of 2,5-dimethoxybenzaldehyde in 220 g nitromethane was treated with 10 g anhydrous ammonium acetate, and heated on a steam bath for 2.5 h with occasional swirling. The deep-red reaction mixture was stripped of the excess nitromethane under vacuum, and the residue crystallized spontaneously. This crude nitrostyrene was purified by grinding under IPA, filtering, and air-drying, to yield 85 g of 2,5-dimethoxy-beta-nitrostyrene as a yellow-orange product of adequate purity for the next step. Further purification can be achieved by recrystallization from boiling IPA.
In a round-bottomed 2 L flask equipped with a magnetic stirrer and placed under an inert atmosphere, there was added 750 mL anhydrous THF, containing 30 g LAH. There was then added, in THF solution, 60 g 2,5-dimethoxy-beta-nitrostyrene. The final solution was a dirty yellow-brown color, and it was kept at reflux temperature for 24 h. After cooling, the excess hydride was destroyed by the dropwise addition of IPA. Then 30 mL 15% NaOH was added to convert the inorganic solids to a filterable mass. The reaction mixture was filtered and the filter cake washed first with THF and then with MeOH. The combined mother liquors and washings
were freed of solvent under vacuum and the residue suspended in 1.5 L H2O. This was acidified with HCl, washed with with 3x100 mL CH2Cl2, made strongly basic with 25% NaOH, and reextracted with 4x100 mL CH2Cl2. The pooled extracts were stripped of solvent under vacuum, yielding 26 g of oily residue, which was distilled at 120-130 °C at 0.5 mm/Hg to give 21g of a white oil, 2,5-dimethoxy-phenethylamine (2C-H) which picks up carbon dioxide from the air very quickly.
To a well-stirred solution of 24.8 g 2,5-dimethoxyphenethylamine in 40 mL glacial acetic acid, there was added 22 g elemental bromine dissolved in 40 mL acetic acid. After a couple of min, there was the formation of solids and the simultaneous evolution of considerable heat. The reaction mixture was allowed to return to room temperature, filtered, and the solids washed sparingly with cold acetic acid. This was the hydrobromide salt. There are many complicated salt forms, both polymorphs and hydrates, that can make the isolation and characterization of 2C-B treacherous. The happiest route is to form the insoluble hydrochloride salt by way of the free base. The entire mass of acetic acid-wet salt was dissolved in warm H2O, made basic to at least pH 11 with 25% NaOH, and extracted with 3x100 mL CH2Cl2. Removal of the solvent gave 33.7 g of residue which was distilled at 115-130 °C at 0.4 mm/Hg.
The white oil, 27.6 g, was dissolved in 50 mL H2O containing 7.0 g acetic acid. This clear solution was vigorous stirred, and treated with 20 mL concentrated HCl. There was an immediate formation of the anhydrous salt of 2,5-dimethoxy-4-bromophenethylamine hydrochloride (2C-B). This mass of crystals was removed by filtration (it can be loosened considerably by the addition of another 60 mL H2O), washed with a little H2O, and then with several 50 mL portions of Et2O. When completely air-dry, there was obtained 31.05 g of fine white needles, with a mp of 237-239 °C with decomposition. When there is too much H2O present at the time of adding the final concentrated HCl, a hydrated form of 2C-B is obtained. The hydrobromide salt melts at 214.5- 215 °C. The acetate salt was reported to have a mp of 208-209 °C.
DOSAGE: 12 - 24 mg.
DURATION: 4 - 8 h.
>> If someone tried to make this synthesis and can come up with pictures and more details would be nice, or even a video if I don't ask too much, I think many would be interested.