Brain
Expert Pharmacologist
- Joined
- Jul 6, 2021
- Messages
- 264
- Reaction score
- 292
- Points
- 63
Psilocybin (3-[2-(Dimethylamino)ethyl]-1H-indol-4-yl dihydrogen phosphate) is an alkaloid of a tryptamine family, phosphorylated psilocin derivative, which has psychedelic properties. Out of all biological sources of psilocybin, mushroom species of genera Psilocybe, Panaeolus, Stropharia, Gymnopilus, Inocybe contain the largest amount; however, about 200 other mushroom species contain psilocybin. A mushroom family containing psilocybin, which is well-known under the name of “magic mushrooms”, has been used for its hallucinogenic effects throughout history. At the end of the 1950s Albert Hoffman from a company Sandoz Laboratories isolated and synthesized psychoactive compounds psilocybin and psilocin from psilocybin-containing fungi. Psilocybin was sold by Sandoz as indocybine for fundamental psychopharmacological and therapeutic clinical trials. Currently, species of Psilocybe are known in Asia, Australia, United States, Canada, Mexico, Central and South America, Africa, and Europe. There is a great level of evidence that the psilocybin containing species began in Africa and Europe, as well as indication that Psilocybe was present in the Old World before the emergence of modern humans. Psilocybin-containing mushrooms may be found in the wild or grown in a controlled environment from spore prints. Psilocybin's popularity grew rapidly in 1960-s. In 1970, it was listed as Schedule I, which led to a significant cut in research on psilocybin. However, recent preliminary studies on psilocybin have shown the prospects of its application in treatment of obsessive-compulsive disorder, alcohol addiction, major depressive disorder and depression in patients with terminal cancer. Psilocybin is classified as a Schedule-I substance in the United States under the Controlled Substances Act of 1971; thus, only limited quantities may be produced each year. Despite its Schedule-I status, psilocybin has been a popular recreational drug since the 1960s and while its use has waned since it became a controlled substance, recreational use continues. Most other developed countries have classified psilocybin and psilocybin-containing mushrooms as illegal as well. The major exception to this generality being The Netherlands, which has a legal loophole allowing the cultivation, sale, and ingestion of psilocybin-containing psychoactive “truffles.” Early evidence of use by Central and South American shamans has been identified in numerous locations. Modern study began in the late 1950s with ethnomycologist R. Gordon Wasson and continued with famed psychedelic researchers Timothy Leary, Ralph Metzner and Ram Dass at Harvard University, Albert Hofmann at Sandoz Labs, Terrence McKenna, and Jonathan Ott in the 1960s and early 1970s. Interest in psychiatrists and psychologists in the 1950s ensued due to its perceived potential as a tool in shortening psychotherapy. Research interested in psychedelic treatment of addiction began as early as the 1950s. Often insightful effects were observed and aided in sobriety, prompting Humphry Osmond, to coin the term “psychedelic” as a way to describe the “mind-manifesting” capabilities of this class of drugs.” Most clinical research was performed in the 1960s, often using the synthetic version indocybin.
Synthesis of Pcilocybin
Synthesis of Pcilocin
Psilocybin has been found in over 100 species of mushroom, many of which are within the genus Psilocybe. The alkaloid psilocybin in the family Inocybeacede appears between 10 and 20 mya, and it is likely that the appearance of psilocybin in the family Psilocybe appeared around that time too. Currently, species of Psilocybe are known in Asia, Australia, United States, Canada, Mexico, Central and South America, Africa, and Europe. There is a great level of evidence that the psilocybin containing species began in Africa and Europe, as well as indication that Psiloche was present in the Old World before the emergence of modern humans. Psilocybin-containing mushrooms may be found in the wild or grown in a controlled environment from spore prints, which are created by placing the cap of a known mushroom on a sheet of wax paper and allowing the spores to fall onto the paper, creating a unique mushroom fingerprint. While the latter is significantly more common, and much safer, some users still seek 'magic mushrooms' out in the wild. The danger of misidentification is ever present and is an error that even the most experienced mycologists are susceptible to. Misidentification can lead to an error that even the most experienced mycologists are susceptible to. Misidentification can lead to anything from mild discomfort to death. Abrupt death is most often seen in amateur mycologists searching for psilocybin-containing mushrooms and another type of psychoactive mushroom commonly known as "Fly Agaric" (Amanita muscaria), which is the iconic red and white dotted mushroom cap often seen in fairytales (Instead of psilocybin, A. muscaria contains the psychoactive drugs muscimol and ibotenio acid.). Unfortunately, several Amanita species are deadly, including the aptly named "Death Cap" (Amanita phalloides) and "Destroying Angel" (Amanita virosa), which can appear very similar to Amanita muscaria and related species. The legacy and use of ‘magic mushrooms’ is seen throughout early history and continues into the modern era. Early evidence of use by Central and South American shamans has been identified in numerous locations. Modern study began in the late 1950s with ethnomycologist R. Gordon Wasson and continued with famed psychedelic researchers Timothy Leary, Ralph Metzner and Ram Dass at Harvard University, Albert Hofmann at Sandoz Labs, Terrence McKenna, and Jonathan Ott the 1960s and early 1970s. Interest in psychiatrists and psychologists in the 1950s ensued due to its perceived potential as a tool in shortening psychotherapy. Research interested in psychedelic treatment of addiction began as early as the 1950s. Often insightful effects were observed and aided in sobriety, prompting Humphry Osmond, to coin the term “psychedelic” as a way to describe the “mind-manifesting” capabilities of this class of drugs.
The structure of psilocybin and other indolealkylamine hallucinogens is similar to endogenous neurotransmitter serotonin, hormone melatonin and supposed endogenous psychedelic N, N-dimethyltryptamine. All of them derive from the same compound – tryptamine. Psilocybin (O-phosphoryl-4-hydroxy-N, N-dimethyltryptamine) and its active dephosphorylated metabolite psilocin (N, N-dimetyltryptamine) structurally belong to the group of tryptamine/indolamine hallucinogens and are structurally related to serotonin. An equimolar dose to 1 mol of psilocin is 1.4 mol of psilocybin. Substitution of the indole nucleus in position 4 probably plays a substantial role in its hallucinogenic effects. Psilocybin and psilocin in their pure forms are white crystalline powders. While psilocybin is soluble in water, psilocin on the other hand is more lipid-soluble. However, psilocin can also be diluted in an acidified aqueous solution and in dimethylsulfoxide (DMSO; up to 100 mM). Furthermore, both substances are soluble in methanol and ethanol, but almost insoluble in petroleum ether and chloroform. Both drugs are unstable in light (in particular in the form of solutions), their stability at low temperatures in the dark under an inert atmosphere is very good. Psilocybin is a thermolabile compound, soluble in water, methanol, ethanol, but is insoluble in organic solvents. It has extremely low activity on its own; it acts mainly as a prodrug of psilocin. Values of pKa range from 1,3 to 6,5. When exposed to ultraviolet light, its stability in solution is disrupted, which causes oxidation. Soluble in 120 parts boiling methanol; difficultly soluble in ethanol; practically insoluble in chloroform, benzene, vapor pressure - 1.9X10-12 mm Hg at 25 °C. Molecular weight is 285,25; it has ammoniac taste, melting point of 224 degrees Celsius, pH 5,2 in 50% water ethanol, TDLo 75 mkg/kg when administered intramuscularly in human, TDLo 60 mkg/kg when administered orally in human, LD50 280 mg/kg when administered intravenously in rats. Psilocybin is considered to poorly penetrate the blood-brain barrier, compared to the psilocin. This is due to the difference in hydrogen bonds, which make the latter more lypophylic facilitating passage through the blood-brain barrier. On visual examination, purified psilocybin and psilocin are distinguishable. One has the appearance of white or almost white needle crystal, the other forms an oily dark brown to black color.
Legal status.
The government enacted a ban on the possession of psilocybin and psilocin in 1968. In 1970, psilocybin and psilocin were listed as Schedule I drugs. In the US, Denver, Oakland, Chicago, Ann Arbor, and Santa Cruz have decriminalized the possession of magic mushrooms, but selling mushrooms is still prohibited. In 2020, Oregon legalized psilocybin, and the District of Columbia decriminalized the use of magic mushrooms. It is illegal to sell and transport psilocybin from Austria. Possession has been decriminalized, and cultivation is legal as long as the mushrooms are not intended for consumption. Those caught with possession of mushrooms intended for personal use may be required to undergo free therapy. In Portugal, psilocybin mushrooms are illegal but decriminalized. Individuals caught with amounts intended for personal use may be required to go to rehabilitation or therapy. In the Netherlands, psilocybin mushrooms are legal in the form of truffles. Consistent with UN Policy, psilocybin is illegal in Italy; however, psilocybe mushrooms are decriminalized. Grow kits and spores are legal to sell and obtain, but administrative punishments such as losing your driver’s license may be the consequence of getting caught with mushrooms. Spain decriminalized personal possession and consumption of psilocybin mushrooms, whereas psilocybin itself remains illegal. Cultivation and sale is illegal, and the legality of spores and grow kits is vague. The British Virgin Islands allows possession and cultivation of psilocybin mushrooms. Although it is illegal (but unenforced) to sell or transport them. Possession, sale, transport, and cultivation of psilocybin mushrooms are all legal in Jamaica. Jamaica openly sells psilocybin mushrooms. It is legal to possess, sell, transport, and cultivate magic mushrooms in Brazil. Psilocybin and psilocin are listed as illegal, however, the mushrooms themselves are not considered illegal. Possession of magic mushrooms is illegal in Mexico, although there’s no enforcement if the magic mushrooms are in indigenous cultures. Cultivation of mushrooms is illegal unless the mushrooms are grown in the wild. Sale and transport are illegal. Magic mushrooms are legal in Samoa. As of 2018, they are illegal in Vietnam.
Currently, classified as a Schedule I substance, researchers at Johns Hopkins University claim that psilocybin mushrooms should be downgraded from a Schedule I to Schedule IV substance. In 2019, the City of Oakland, CA voted to decriminalize magic mushrooms just one month after Denver, CO decided to do so. There is a push in California to decriminalize psilocybin mushrooms on a statewide level, with many psychedelic awareness and advocacy groups supporting this change. Moreover, Oregon’s state legislature is considering passing a bill to decriminalize psilocybin mushrooms and offer licenses to grow. Much of this promising research is happening because of the Multidisciplinary Association of Psychedelic Studies (MAPS).MAPS is currently doing medical research with MDMA, psilocybin, LSD, marijuana, ibogaine, and ayahuasca. Another organization, Heffter Research Institute specializes in psilocybin research. Among Heffter Research Institute’s board of directors is co-founder Dr. David Nichols, a widely celebrated researcher, professor, and expert on the chemistry of hallucinogens. Other researchers include Dr. Dennis McKenna, an esteemed writer, ethnopharmacologist, psychonaut, and brother to the late Terrence McKenna, and Dr. Roland Griffiths who is doing phenomenal research at Johns Hopkins into psilocybin and addiction. There are also several psilocybin studies currently underway internationally. The Beckley Foundation, based in the UK, is sponsoring some of the most progressive psilocybin research around today. Founded in 1996 by Amanda Fielding—an esteemed psychonaut and scientist herself—the Beckley Foundation is currently sponsoring research on the neuroscience of psilocybin, along with MAPS and Heffter. This research is taking place at Imperial College London with Dr. David Nutt and Dr. Robin Carhart-Harris, both of whom have published psilocybin research in the past, namely the “Entropic Brain Model.” Heffter is also sponsoring a study in Switzerland entitled “Psilocybin Effects on Attention, Perception, and Cognition.” Similarly, MAPS is sponsoring a study called “Experimental Studies on the Effects of Psilocybin on Binocular Depth Inversion, Binocular Rivalry” in Germany.
Pharmacokinetics and pharmacodynamics.
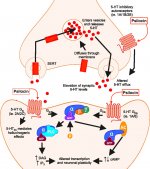
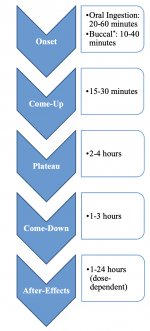
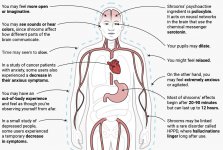
When ingested, psilocybin undergoes biotransformation in the liver, where it is dephosphorylated to psilocin by an unknown enzyme. After psilocin has entered the systemic blood flow, it enters the brain, where it has psychoactive effect. According to other data, after oral administration, psilocybin is rapidly dephosphorylated in the acidic environment of the stomach or under the action of alkaline phosphatase (and other nonspecific esterases) in the intestine, kidneys and possibly in the blood with the formation of a phenolic compound psilocin, which can easily pass through the blood-brain barrier. It is noteworthy that the relative potency of psilocin to psilocybin is almost identical to the ratio of the molecular weight of these compounds. Blocking of alkaline phosphotase by competitive β-glycerophosphate substrates levels symptoms of intoxication. Psilocin undergoes both phases of metabolism. First phase metabolism includes oxidative deamination of psilocin to 4-hydroxyindole-3-acetaldehyde catalyzed by liver monoamine oxidase or aldehyde dehydrogenase with consequent oxidation to 4-hydroxy-indole-3-acetic acid, 4-hydroxy-indole-3-acetaldehyde and 4-hydroxytryptophole. The enzymes participating in this processes haven't been identified. That is why MAO inhibitors can enhance hallucinogenic effects of psilocybin, just like ethanol can enhance the trip because its primary metabolite acetaldehyde reacts in vivo with endogenous amines, as a result, MAO-inhibitors tetrahydroisoquinolines and β-carbolines are formed. Since psilocybin induces competitive inhibition of MAO (which metabolises serotonin), serotonin level in the brain increases and simultaneously 5-HIAA concentration is decreased. Also, there is insignificant in terms of clinical effects metabolic pathway, which involves oxidation resulting in the formation of dark blue product, which has the structure of o-quinone or iminoquinone. This pathway is catalyzed by hydroxyindol oxidases (ceruloplasmin, the copper containing oxidase of mammalian plasma, and cytochrome oxidase). Above metabolites demonstrate insignificant physiological activity. When psilocybin is administrated parenterally, tissue phosphatases have the same role, and the ones in kidneys are among the most active. Considering that the competitive blockade of dephosphorylation blocks psychotropic action of psilocybine, it is clear that psilocin is the main active metabolite of psilocybin. Within 5 hours since oral administration of psilocybin, up to 80% of psilocin is present in blood as a conjugate O-glucuronide and is excreted in urine unchanged. Glucuronidation of hydroxyl group to psilocinO-glucuronide is an important stage of detoxification, that is why enzymatic hydrolysis increases the time of detection in urine samples. While psilocin undergoes extensive glucoronidation by UDP-glucuronosyltransferases (UGT)1A10 in small intestine, UGT1A9 makes the main contribution to glucoronidation after its absorption into the blood. N-glucuronidation doesn't occur throughout metabolism in this case. Besides the pathway mentioned above, psilocin itself undergoes oxidative metabolism as well. Demethylation and desamination of 4-hydroxyindol-3-yl-acetaldehyde (4-HIA) with subsequent oxidation (presumably by hepatic aldehyde dehydrogenase and monoamine oxidase) results in the formation of 4-hydroxyindol-3-acetic acid (4-HIAA) and 4-hydroxytryptofol (4-HT). These insignificant metabolites (about 4% disintegrates in the way described above) can be detected in human blood plasma. The third way of metabolism of psilocin involves oxidation by hydroxyindol oxidases as it was stated above. Psilocin is distributed to all the tissues, including the brain, and it is eliminated within 24 hours. Most part is eliminated within the first 8 hours (about 65% in urine and 15-20% with bile and feces). It can be detected in urine during 2 weeks. The highest concentrations of psilocin are in neocortex, hippocampus, extrapyramidal motor system and reticular formation. In humans, psilocybin and psilocin can be detected in blood plasma after 20-40 minutes since oral administration. Maximum concentrations are reached in 80-100 minutes and can be detected within six hours. Half-life period of psilocin in blood plasma is about 2/5 hours after oral administration, and 1.23 hours – after parenteral administration. As it has been mentioned already, about 80% of psilocin in plasma is in conjugated form. Psilocin (90-97%) and psilocybin (3-10%) can be detected in urine unchanged or conjugated with glucoronic acid. Elimination half life is about 50 minutes, elimination constant is 0.307/h. Most of the substance is eliminated within the first three hours after oral administration and is completely excreted in the urine within 24 hours. The complete metabolic pathway of psilocybin has been studied very little, and there is still much information that must be gathered to determine the exact mechanisms involved in its metabolism.
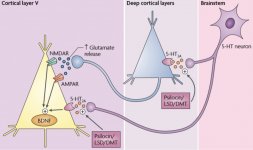
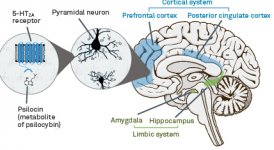
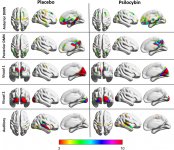
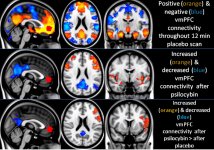
Pharmacology of psilocybin is very complex and has been insufficiently studied. Psilocybin can presumably have its own insignificant activity; however, it mostly acts as a prodrug of psilocin. The latter easily passes through the blood-brain barrier and has its psychoactive effect there. The main binding sites are summarized in the table above. Psilocin has the strongest binding to serotonin receptors: 5-HT1D,2B,2C,5,6,7), it also has a moderate binding potential to serotonin receptor sites(5-HT1A, 1B, 2A). Aside from serotonin receptors, psilocin has a certain affinity to histamine receptors of the first type (H1), alpha-2A and -2B receptors, and to dopamine receptors of the third type (D3). In neurons expressing the 5HT2A receptor, but not in 5HT2A knockouts, psilocybin increases the expression of early genes (erg-1, erg-2, c-fos, jun-B, period-1, gpcr-26, fra-1, N- 10, I-κBα) and reduces the expression of sty-kinase. Needless to say, the precise signaling pathway leading from the receptor to the activation of early genes is not yet known. Given that a non-hallucinogenic lisuride also activates the c-fos, it is likely that the expression of c-fos only reflects increased neuronal activity, while the expression of egr-1/ egr-2 is specific for the hallucinogenic effect. Gonzales-Meaeso explained this selectivity with the “agonist trafficking of receptor signaling theory”, where hallucinogens activate the 5HT2A/mGlu2 receptor heterocomplex and different G proteins compared to non- halucinogenic 5-HT2A agonists. This hypothesis is supported in a study where mice with the knockout gene for the mGlu2 receptor do not display any head twitch behavior. Psilocilin has been proven to inhibit sodium-dependent serotonin transporter (SERT), which leads to an increase in serotonin concentration. Serotonin stays in the synaptic cleft after its release, which in the end leads to repeated activation of serotonergic postganglionic neurons. As for the assessment of binding affinity to receptors, they are ordered as follows: 5HT2B > 5HT1D > D1 > 5HT1E > 5HT1A > 5HT5A > 5HT7 > 5HT6 > D3 > 5HT2C > 5HT1B > 5HT2A. Binding to imidazoline receptors of the first type has been proved as well. It is interesting to note, that psilocybin affinity to human 5-НТ2А receptors is 15 times higher than that of rats. Agonism of 5НТ2А receptors and activation of excessive number of these receptors and receptor subtypes are responsible for the unique and intense psychedelic effects caused by psilocybin. Earlier, there have been a lot of discussions about whether 5-НТ2А or 5-НТ2С receptors are responsible for isolated hallucinations after ingestion of psilocybin-containing mushrooms. The studies have shown that 5-НТ2А-antagonists suppress hallucinations, whereas antagonists of 5-НТ2С have neither the potentiation of hallucinations, nor the leveling effect on them. Thus, agonism to 5-НТ2А receptors is associated with general neuron excitation, improvement of memory and learning, contractions of vascular smooth muscle tissue, gastrointestinal tract and bronchi, certain anti-inflammatory activity, increased production of prolactin and oxytocin, adrenocorticotropic hormone and renin on activation of the renin-angiotensin-aldosterone system. As for the 5-НТ2С activation by psilocin, activation of proopiomelanocortin (the precursor of α-, β-, and γ-melanocyte stimulating hormone and adrenocorticotropic hormone) and release of cortisol occurs. These hormones provide increased appetite, insulin sensitivity, glucose metabolism, stabilization to anxiogenic and stressful stimuli. Psilocin acts as a partial agonist of 5-НТ1А receptors, which are mainly expressed in raphe nucleus (DRN) and median raphe nucleus (MRN), located near the midline of the brainstem along its entire rostro-caudal extension, as somatodendritic autoreceptors. MRN promotes the activation of memory consolidation processes and is projected to the hippocampus, while VRN is one of the largest serotonergic nuclei in the human brain, which provides a significant amount of serotonergic innervation of the forebrain; in addition, MRN has projection fibers in the amygdala and hypothalamus, which is associated with regulation of circadian rhythm and of several types of cells that produce catecholamine and substance-P. DRN and MRN are rich in presynaptic 5-НТ1А receptors, and psilocin has a number of times (5-6 times) stronger effect particularly on its presynaptic sites compared to postsynaptic. This preference is explained by a high density of 5-НТ1А receptors, which are located in these areas. This certain type of receptors, located on the bodies of serotonergic cells of the raphe zone, is not found, for example, on postsynaptic membranes. In studies by fMRI, it was revealed that psilocybin significantly decreases blood flow and venous oxygenation in the brain. This fact correlates with its subjective effect and significantly reduces the positive connection between two key structural nodes (mPFC и PCC). Psilocybin is proved to increase glucose metabolism in the brain. Also, it has been proved by some experiments that DMN is also crucial to maintain cognitive integration and limitations under normal conditions. After psilocin binding to presynaptic 5-НТ1А receptors of DRN area, it suppresses the effects of this area, while the underlying cells remain intact and enhance sympathetic activity associated with locus coeruleus. Other localizations of 5-НТ2А receptors demonstrate a rapid decrease in the activity of this receptor and a decrease in their density on activation by psilocin. So, psilocybin has no tolerance to inhibiting action of DRN. It is important to note, that selective agonists of 5-НТ1а receptors are not hallucinogenic at their core, however, they have a role in influencing inhibitory effects, which are identified in DRN.
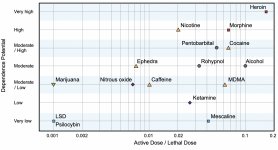
Though dopamine receptor of the second type plays a significant role in hallucinations forming in various mental illnesses, it is assumed that it doesn't have any active and indirect relationship to psilocin action. This hypothesis was first proved by Vollenweider et al., when they found that galoperidol administration (agonist of D2R) doesn't weaken the psychoactive effects of psilocin. Even though dopaminergic effects of psilocin are considered to be minimal, it has relatively high binding ability to D3-receptor, compared to other dopamine receptor subtypes. Despite the fact that the effects mediated through D3R have been little studied, it presumably contributes to the characteristic psychoactive properties of psilocybin and its ability to cause addiction. The chemical precursor of psilocin, 4-acetoxy-N,N-dimethyltryptamine, has the phosphoryloxy group replaced by an acetoxy group. It is metabolized in the same way as the phosphoryloxy group, and this modification allows a way around some metabolic processes of the first phase. Despite psilacetin being identical pharmacological substitution to psilocybin, a lot of users report that it has some insignificant but distinguishable difference between them. Psilacetin is often described as a substance with more rapid onset of action, which doesn't involve anxiety and nausea (which are associated with psilocybin use due to lack of chitin, usually contained in the mushrooms) and has shorter duration. It is well known that median lethal dose in rats is about 293 mg/kg, which indicates a huge therapeutic window of psilocin. Tachyphylaxis, rapid desensitization to a substance, leading to a decrease in the physiological effect is a phenomenon, associated with the use of many hallucinogens. Tolerance to psilocybin starts to develop right after the first single use. The mechanism involves physiological response to excessive stimulation of 5-НТ2А receptors by rapid reduction of receptor sites and a decrease in the density of receptors on the cell. In general, it is believed that these receptor sites return to fifty percent of the original level in 3-7 days since the initial use. They return to their original amount in 4 weeks, depending on the dose and duration of repeated use. Besides, there is cross-tolerance between indolealkylamine and phenylalkylamine classes of hallucinogens.
Attachments
-
 aDjgnEic18.jpg572.2 KB · Views: 1,591
aDjgnEic18.jpg572.2 KB · Views: 1,591 -
 sRHGpjI9LU.jpg788.9 KB · Views: 1,561
sRHGpjI9LU.jpg788.9 KB · Views: 1,561 -
 TQv018fRJh.jpg128.6 KB · Views: 922
TQv018fRJh.jpg128.6 KB · Views: 922 -
lF1rOfpW8k.jpg2.6 MB · Views: 948
-
 TSkYz5Gq6P.jpg363.8 KB · Views: 1,718
TSkYz5Gq6P.jpg363.8 KB · Views: 1,718 -
 sPlo2QDBMe.jpg551.6 KB · Views: 1,688
sPlo2QDBMe.jpg551.6 KB · Views: 1,688 -
 AxRV2Hfutv.jpg697.8 KB · Views: 1,710
AxRV2Hfutv.jpg697.8 KB · Views: 1,710 -
 b2Ma0wsVTD.jpg607.2 KB · Views: 1,775
b2Ma0wsVTD.jpg607.2 KB · Views: 1,775 -
 QtXjIHo7PJ.jpg438.5 KB · Views: 1,580
QtXjIHo7PJ.jpg438.5 KB · Views: 1,580 -
 rI0YMKgE6z.jpg1.1 MB · Views: 1,719
rI0YMKgE6z.jpg1.1 MB · Views: 1,719 -
 Q1ueUSGlFN.jpg614.4 KB · Views: 1,723
Q1ueUSGlFN.jpg614.4 KB · Views: 1,723
Last edited by a moderator:
