G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,704
- Solutions
- 3
- Reaction score
- 2,849
- Points
- 113
- Deals
- 1
Introduction
BB Forum team glad to represent elementary and simple ADB-PINACA synthesis, which is not require rare or elaborated equipment. There is step-by-step video guide and clear write up about ADB-PINACA formation with a good yield. This synthesis can be repeated even by chemistry beginners.Equipment and glassware:
- Flat bottom flask 2 L;
- Funnel;
- pH indicator paper;
- Ice water bath;
- Retort stand and clamp for securing apparatus (optional);
- Glass rod;
- Measuring cylinder 1 L;
- Laboratory scale (1-200 g is suitable);
- Beakers 5 L; 2 L; 1 L x2; 500 ml x3;
- Separatory funnel 2 L;
- Plastic or ground-glass plugs;
- Magnetic or top stirrer;
- Plastic spoon;
- Separatory funnel 2 L;
- Pressure equalized drip funnel (500 ml);
- Methyl 1H-indazole-3-carboxylate 50 g;
- Anhydrous DMF 800 ml;
- Sodium hydride (NaH) 60% in mineral oil 16 g;
- 1-Bromopentane 43 g;
- Brine (NaCl) solution;
- Ethyl alcohol 800 ml;
- Alkali solution (NaOH 17 g in water 400 ml);
- Distilled water;
- L-tert-leucinamide hydrochloride 52 g;
- Hydroxybenzotriazole hydrate (HOBT*H2O) 48 g;
- 1-Ethyl-3-carbodiimide (EDC*Hcl) 82 g;
- Triethylamine 100 g;
Difficulty rating: 2/10
Stage I. Preparation of Methyl 1-Pentyl-1H-indazole-3-carboxylate
1. Methyl 1H-indazole-3-carboxylate 50 g is poured into the 2 L flask.2. DMF 300 ml is added.
3. Methyl 1-pentyl-1H-indazole-3-carboxylate has to be completely dissolved with stirring. This solution should be cooled down in a freezer to ~5℃ or an ice water bath (optional).
4. Sodium hydride (NaH) 60% in mineral oil 16 g is added in small portions. The solution is stirred until a complete dissolution. Make sure that reaction temperature doesn’t rise much (not higher than room temperature). If NaH is added without cooling, the addition procedure will take very long time.
Note: Glassware used in this reaction must be dry. DMF must be anhydrous. All used equipment must be completely dry. After the end of NaH addition, the mixture is brought to room temperature (if the reaction was cooled) or stirred (if it wasn't cooled) for an additional hour.
5. After a while, the mixture is cooled down again to ~5-15℃. If the mixture isn't cooled, then adding the next component will take much more time.
6. 1-Bromopentane 43 g is added dropwise with constant stirring. Then, the mixture is stirred for 1-2 days.5. After a while, the mixture is cooled down again to ~5-15℃. If the mixture isn't cooled, then adding the next component will take much more time.
7. A brine solution is poured into the mixture. The brine is saturated salt (NaCl) solution in water, at least 300 ml or more, up to the capacity of the reaction flask.
8. Layers are separated. The separated 1-pentyl-1H-indazole-3-carboxylate is collected.
The reaction yield is 70 g (99.8%).
Stage II. 1-Pentyl-1H-indazole-3-carboxylic Acid Intermediate Preparation
9. The resulting Methyl 1-pentyl-1H-indazole-3-carboxylate 70 g is added into the reaction flask. There is no requirement for an anhydrous condition.10. Ethyl alcohol 400 ml is added and the mixture is stirred. Methyl alcohol can be used instead.
11. An alkali solution (NaOH 17 g in water 400 ml) dropwise while stirring.
12. Another 400 ml portion of alcohol is added while stirring until the mixture becomes transparent.
Note: The volume of addition alcohol portion depens on solvent (EtOH/MeOH/IPA) and the alkali solution concentration. It is possible to take isopropyl alcohol or various alkalis.
13. The mixture is stirred for 2 days.
14. Then, the mixture is diluted with water (as much as the reaction vessel allows) and acidified with hydrochloric acid to pH 2.13. The mixture is stirred for 2 days.
15. A resulting layer is separated from water as soon as possible, before a crystallization start.
The reaction yield is 50 g (75%).
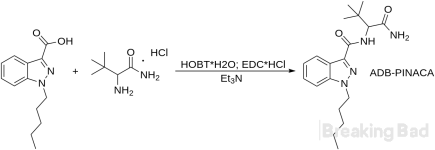
Stage III. ADB-PINACA From 1-Pentyl-1H-indazole-3-carboxylic Acid
16. DMF 500 ml is added to the resulting 1-pentyl-1H-indazole-3-carboxy acid 50 g, the mixture is stirred.17. Next, L-tert-leucinamide hydrochloride 52 g is added and stirred until complete dissolution.
18. Then, hydroxybenzotriazole hydrate (HOBT*H2O) 48 g is poured in the mixture and stirred until complete dissolution.
19. Following step, 1-ethyl-3-carbodiimide (EDC*Hcl) 82 g is added and stirred until complete dissolution.
20. Triethylamine 100 g is poured into the drip funnel and added dropwise with constant stirring (the mixture is turning cloudy).
21. The reaction mixture is stirred for one day.
22. The brine is added (at least 1 L), the mixture is diluted with more water volume in a larger vessel and stirred. Water is added until an oil layer separation.
23. The ADB-PINACA oil is formed on the solution surface after stirring, it is collected manually. If the oil is left for a long time, it will crystallize right there.
ADB-PINACA yield is 40 g.
24. The ADB-PINACA oil residues on the used containers are rinsed with alcohol. ADB-PINACA is easily dissolved in alcohol at room temperature. This alcohole (from rinsings) is used for ADB-PINACA recrystallization.
25. The mixture of alcohol and ADB-PINACA oil is heated until complete dissolution.
Note: A small amount of insoluble salt may be precipitated, this is a side product and should be separated from the solution.
26. The solution is left in a warm air to crystallize during evaporation or placed into a freezer.
Note: It’s worth to place ADB-PINACA into silicon baking forms and let crystallize on warm air flow until all solvent evaporation.
26. The solution is left in a warm air to crystallize during evaporation or placed into a freezer.
Note: It’s worth to place ADB-PINACA into silicon baking forms and let crystallize on warm air flow until all solvent evaporation.