- Joined
- Jan 1, 2022
- Messages
- 44
- Reaction score
- 52
- Points
- 18
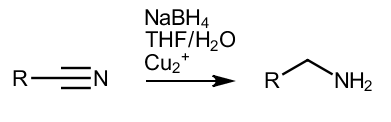
For small batch reduction of P2NP, this method is faster and safer than using aluminum foil and mercury salts, and there are no toxic mercury salts in the reaction,and the yield of this reaction is relatively high
1-phenyl-2-nitropropene - 5 g (0.031 M, 1 eq), m.p. 65-66C
NaBH4 - 8.5 g (0.225 M, 7.2 eq)
THF - 120 ml
H2O - 60 ml
CuCl2 * 2 H2O - 0.53 g (0.0031 M, 0.1 eq), solution in 3 ml H2O
A 500 ml three-necked flask equipped with adequate RO, addition funnel and thermometer is charged with 80 ml of THF and 60 ml of water. NaBH4 was then added to the flask all at once, 7.2 equivalents, 8.5 g, stirring was turned on, and the flask was placed in an ice bath of snow and water. The nitroalkene, dissolved in 40-45 ml of THF, was carefully added via addition funnel, and after about half of the solution had been added, the flask was removed from the ice bath and the rate of addition of THF+RNO2 was increased. By the end of the addition, the temperature in the flask, the contents of which turned white, was about 45-50C - and, without thinking twice, a solution of 0.53 g CuCl2 / 3 ml H2O was added from a syringe through a reflux condenser. Copper chloride was added in one portion, very quickly, and Pm instantly turned black with a violent evolution of hydrogen. The temperature continued to be in the region of 50C - despite the obvious exotherm that took place inside. The released hydrogen thus cools Pm due to its "evaporation". The flask was then gently heated until the thermometer read 64-66C. At this moment, firstly, the active evolution of hydrogen stops and THF itself begins to boil. At the same time, secondly, Pm is clearly divided into 2 phases - an almost transparent upper layer and a lower layer black from copper. Swim thinks that a rise in temperature from 45-50C to 65C (Bp THF) is a very convenient indicator of the progress of a reaction.
Then Rm was cooled in an ice bath to 20C, and 15-20 ml of 37% hydrochloric acid was added through an addition funnel - no active reaction was observed, therefore, all the NaBH4 was consumed in the reduction process. After that, the flask was again cooled to 15–20°C, and 30–35 g of NaOH in ~150 ml of water were added through the same dropper. After a short stirring, Rm again divided into layers - and the lower, inorganic, became orange in color (as I understand it, the Cu-NH coordinate complex collapsed and copper was released in free form). And most importantly, there was a minimal amount in the organic phase of copper. This, of course, is by eye. Just in case, edible salt was added to Rm, and all the contents were poured into a separating funnel. The lower layer was extracted with a mixture of MTBE + THF (25+25 ml), the extract was combined with the main layer of THF and diluted with 40 ml of MTBE. After that, the organics were washed with 15% NaOH solution and saturated NaCl solution, dried with Na2SO4, and then neutralized with a weak solution of H2SO4 in CH3CN, diluted with ice-cold acetone, filtered and washed again thoroughly with acetone.
Yield: 4.3 g of 1-phenyl-2-aminopropane as sulfate salt.
1-phenyl-2-nitropropene - 5 g (0.031 M, 1 eq), m.p. 65-66C
NaBH4 - 8.5 g (0.225 M, 7.2 eq)
THF - 120 ml
H2O - 60 ml
CuCl2 * 2 H2O - 0.53 g (0.0031 M, 0.1 eq), solution in 3 ml H2O
A 500 ml three-necked flask equipped with adequate RO, addition funnel and thermometer is charged with 80 ml of THF and 60 ml of water. NaBH4 was then added to the flask all at once, 7.2 equivalents, 8.5 g, stirring was turned on, and the flask was placed in an ice bath of snow and water. The nitroalkene, dissolved in 40-45 ml of THF, was carefully added via addition funnel, and after about half of the solution had been added, the flask was removed from the ice bath and the rate of addition of THF+RNO2 was increased. By the end of the addition, the temperature in the flask, the contents of which turned white, was about 45-50C - and, without thinking twice, a solution of 0.53 g CuCl2 / 3 ml H2O was added from a syringe through a reflux condenser. Copper chloride was added in one portion, very quickly, and Pm instantly turned black with a violent evolution of hydrogen. The temperature continued to be in the region of 50C - despite the obvious exotherm that took place inside. The released hydrogen thus cools Pm due to its "evaporation". The flask was then gently heated until the thermometer read 64-66C. At this moment, firstly, the active evolution of hydrogen stops and THF itself begins to boil. At the same time, secondly, Pm is clearly divided into 2 phases - an almost transparent upper layer and a lower layer black from copper. Swim thinks that a rise in temperature from 45-50C to 65C (Bp THF) is a very convenient indicator of the progress of a reaction.
Then Rm was cooled in an ice bath to 20C, and 15-20 ml of 37% hydrochloric acid was added through an addition funnel - no active reaction was observed, therefore, all the NaBH4 was consumed in the reduction process. After that, the flask was again cooled to 15–20°C, and 30–35 g of NaOH in ~150 ml of water were added through the same dropper. After a short stirring, Rm again divided into layers - and the lower, inorganic, became orange in color (as I understand it, the Cu-NH coordinate complex collapsed and copper was released in free form). And most importantly, there was a minimal amount in the organic phase of copper. This, of course, is by eye. Just in case, edible salt was added to Rm, and all the contents were poured into a separating funnel. The lower layer was extracted with a mixture of MTBE + THF (25+25 ml), the extract was combined with the main layer of THF and diluted with 40 ml of MTBE. After that, the organics were washed with 15% NaOH solution and saturated NaCl solution, dried with Na2SO4, and then neutralized with a weak solution of H2SO4 in CH3CN, diluted with ice-cold acetone, filtered and washed again thoroughly with acetone.
Yield: 4.3 g of 1-phenyl-2-aminopropane as sulfate salt.