- Joined
- Feb 1, 2023
- Messages
- 1
- Reaction score
- 0
- Points
- 1
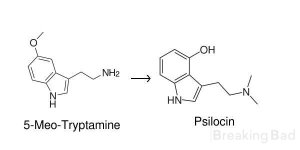
A beginner here. I was wondering if it was possible to synthesize either psilocin or 4-Ace-DMT, starting with 5-Meo-Tryptamine. I'm very confused as to how to create the hydroxyl group of the psilocin's ring from the starting molecule (see the attached image).
Am I supposed to use a redox reaction on 5-MeO and then rebuild the OH side chain somehow? Any help is appreciated, thanks!
Am I supposed to use a redox reaction on 5-MeO and then rebuild the OH side chain somehow? Any help is appreciated, thanks!