- Joined
- May 14, 2023
- Messages
- 7
- Reaction score
- 4
- Points
- 3
To be fair and keeping it on topic, there is only one 3,4-Methylenedioxymethamphetamine (MDMA). There is really no other MDMA in 100% pure form because the name represents a certain molecule. You can argue all you want about impurities affecting effects etc and the synthesis being tricky, and 100% pure MDMA gets rarely sold in illegal circuits, but that's besides the point OP is making.
↑View previous replies…
- Joined
- May 18, 2023
- Messages
- 37
- Reaction score
- 19
- Points
- 8
- Joined
- May 18, 2023
- Messages
- 37
- Reaction score
- 19
- Points
- 8
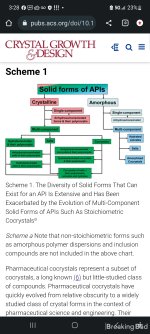
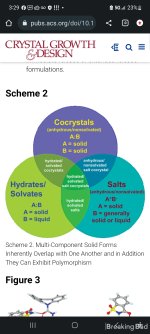
detailed earlier herein, the nature of multicomponent solid forms is such that there is significant overlap between salts, cocrystals, and hydrates (Scheme 2), and this can create difficulties when it comes to classification. With this in mind, the strong consensus of the authors is that cocrystals should naturally be grouped with salts for this and a number of other reasons:
•Given that the difference between a salt and a cocrystal might just be the movement of a proton by around 1 Å, is there any reason why the type of interaction in a solid form should in effect be used to classify it? For example, a formic acid solvate of an API could be classified as a solvate, a cocrystal, or a salt. However, only the nature of the interaction between the two components will tell one how to classify such a molecular complex. Does the nature of this interaction have any relevance at all to pharmaceutical science and clinical performance?
•The issue of the “salt cocrystal continuum”, (17) which was raised by the FDA, has not been studied in sufficient breadth or depth to conclude the frequency or importance of this phenomenon. Furthermore, it becomes moot if salts and pharmaceutical cocrystals are grouped together.
•Like salts, cocrystals have defined stoichiometries, and similar solution speciation characteristics, such as common-component effects (similar to common ion effects of salts), multiple ionization (API and coformer), and association (self-association and complexation).
•Similar to salts, cocrystals will exhibit a solubility product (Ksp) and a pHmax (that specifies the thermodynamic stability region of the cocrystal). (18) These properties are of paramount importance in the performance aspects and analytical procedures that cocrystals will require (such as level of coformer, common components) to provide reasonable assurance of their safety and effectiveness.
•There are already marketed drugs that could be classified as cocrystals. Caffeine citrate, (19) Depakote (the valproic acid cocrystal of sodium valproate), (20) and Escitalopram oxalate are marketed as salts but they could be classified as cocrystals according to our proposed definition.
•Polymorphism in cocrystals (different packing arrangements with the same composition, e.g. carbamazepine: saccharin (21) (Figure 3), piroxicam: 4-hydroxybenzoic acid (11d) and hydrates of cocrystals) (22) defy the idea that cocrystal formers play the same role as that of an excipient. Rather, cocrystals are novel solid forms that can be patented (23) and are known to modulate physicochemical properties such as solubility in either direction
•Given that the difference between a salt and a cocrystal might just be the movement of a proton by around 1 Å, is there any reason why the type of interaction in a solid form should in effect be used to classify it? For example, a formic acid solvate of an API could be classified as a solvate, a cocrystal, or a salt. However, only the nature of the interaction between the two components will tell one how to classify such a molecular complex. Does the nature of this interaction have any relevance at all to pharmaceutical science and clinical performance?
•The issue of the “salt cocrystal continuum”, (17) which was raised by the FDA, has not been studied in sufficient breadth or depth to conclude the frequency or importance of this phenomenon. Furthermore, it becomes moot if salts and pharmaceutical cocrystals are grouped together.
•Like salts, cocrystals have defined stoichiometries, and similar solution speciation characteristics, such as common-component effects (similar to common ion effects of salts), multiple ionization (API and coformer), and association (self-association and complexation).
•Similar to salts, cocrystals will exhibit a solubility product (Ksp) and a pHmax (that specifies the thermodynamic stability region of the cocrystal). (18) These properties are of paramount importance in the performance aspects and analytical procedures that cocrystals will require (such as level of coformer, common components) to provide reasonable assurance of their safety and effectiveness.
•There are already marketed drugs that could be classified as cocrystals. Caffeine citrate, (19) Depakote (the valproic acid cocrystal of sodium valproate), (20) and Escitalopram oxalate are marketed as salts but they could be classified as cocrystals according to our proposed definition.
•Polymorphism in cocrystals (different packing arrangements with the same composition, e.g. carbamazepine: saccharin (21) (Figure 3), piroxicam: 4-hydroxybenzoic acid (11d) and hydrates of cocrystals) (22) defy the idea that cocrystal formers play the same role as that of an excipient. Rather, cocrystals are novel solid forms that can be patented (23) and are known to modulate physicochemical properties such as solubility in either direction
https://pubs.acs.org/doi/10.1021/cg3002948#
Attachments
- Joined
- May 18, 2023
- Messages
- 37
- Reaction score
- 19
- Points
- 8
Not me "maps" During my A-levels (the UK’s version of high school), I made the choice to drop art class and take up chemistry. I had come to really enjoy chemistry during my school years, and eventually it seemed like a better option for me to study than art, which felt too abstract—chemistry is exact and predictable, right? What I learned throughout those years and my subsequent chemistry degree, however, was something different: Science is not exact, and our understanding is constantly evolving. It started to seem like a relentless attempt to place evermore complex sets of round pegs in square holes.
Having said that, it is not as though the results of these human scientific endeavors aren’t astounding and necessary, as they include feats of engineering and medicine. The point is that even those feats are imperfect; they are not always fully understood and can carry risks. Just like the building that won awards for structural and architectural brilliance can have unknown faults and fail years later, the drug that is shown to be highly effective in its target indication can bring unknown side effects that surface after licensure.
This background helps me understand why the chemistry of MDMA, a well-known molecule first developed in 1912 and patented by Merck in 1914, is not exact, not yet fully known, and still requires detailed, stringent analyses and controls.
And why one does not simply easily dissolved in water. I think this last argument is also the strongest against your case. . If people didnt boof it put it in water etc. etc. etc. even after multiple re crystallizations in multuple solvents to the point where MDMA begun looking like pure meth shards but still having issues in water or orally.It has an excellent bio-availability in the 60-70% range kinda flys out the window when I recrystallized MDMA to look like pure meth. With me and many other people it was still strangely still felt lackluster?
The path for a molecule, therefore, from early-phase API and drug product to a commercial product, is an iterative one. It involves increased levels of understanding around the synthesis, potential impurities, isomers, crystalline forms, and physical properties—all of which need to be controlled and understood to the fullest, as they can have an effect the safety and pharmacology of the drug.
The chemistry of MDMA is not a given, and requires expert development to get to the commercial standard we need to ensure patient access and safety at scale. However, it should not be expected that we will stop learning about the chemistry of this compound; changes in manufacturing process, scale, and product formulation can bring with them new challenges and lessons.

 maps.org
maps.org
Having said that, it is not as though the results of these human scientific endeavors aren’t astounding and necessary, as they include feats of engineering and medicine. The point is that even those feats are imperfect; they are not always fully understood and can carry risks. Just like the building that won awards for structural and architectural brilliance can have unknown faults and fail years later, the drug that is shown to be highly effective in its target indication can bring unknown side effects that surface after licensure.
This background helps me understand why the chemistry of MDMA, a well-known molecule first developed in 1912 and patented by Merck in 1914, is not exact, not yet fully known, and still requires detailed, stringent analyses and controls.
And why one does not simply easily dissolved in water. I think this last argument is also the strongest against your case. . If people didnt boof it put it in water etc. etc. etc. even after multiple re crystallizations in multuple solvents to the point where MDMA begun looking like pure meth shards but still having issues in water or orally.It has an excellent bio-availability in the 60-70% range kinda flys out the window when I recrystallized MDMA to look like pure meth. With me and many other people it was still strangely still felt lackluster?
The path for a molecule, therefore, from early-phase API and drug product to a commercial product, is an iterative one. It involves increased levels of understanding around the synthesis, potential impurities, isomers, crystalline forms, and physical properties—all of which need to be controlled and understood to the fullest, as they can have an effect the safety and pharmacology of the drug.
The chemistry of MDMA is not a given, and requires expert development to get to the commercial standard we need to ensure patient access and safety at scale. However, it should not be expected that we will stop learning about the chemistry of this compound; changes in manufacturing process, scale, and product formulation can bring with them new challenges and lessons.

The Commercial Chemistry of MDMA: From Research to Patient Access
Written by Heather Clouting, M.Sc. MAPS Bulletin Spring 2020: Vol. 30, No. 1 Download this article. Nearly 20 years ago, during our final year of university while studying for our Bachelor’s in chemistry, I thought that two fellow students and I were rebels for suggesting that our organic...
 maps.org
maps.org
Last edited:
- Joined
- May 18, 2023
- Messages
- 37
- Reaction score
- 19
- Points
- 8
I also wanna say I dont know crap about this but there has to be something to it that other people understand better then me. and have degrees but the honest truth I think the real pros even people producing 100s of kilos are still missing something maybe nick sand or others might know ... but then again MAPS doesnt even have much more info vs me or others...