You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
LSD synthesis from Ergotamine
- Thread starter G.Patton
- Start date
- Language
- 🇺🇸
- Joined
- Nov 17, 2022
- Messages
- 31
- Reaction score
- 13
- Points
- 8
3 grams of lsd gives 20000 to 30000 doses , excuse my noob question, how did they separate this exact small quantity to blotter on 30000 doses?
Tnx for any reply
Tnx for any reply
Boss I buy a LSD solution, 100mg in 5ml of vodka and i want to store indefinitely. Have some questions..
-Is better in the freezer or in the fridge?
-Is neccesary to bubble argon or nitrogen to the solution and fill the empty top part of the dropper with the inert gas or vaseline or something like that?
-Put a few mg of EDTA?
Thanks!!!
I want to store for years.
-Is better in the freezer or in the fridge?
-Is neccesary to bubble argon or nitrogen to the solution and fill the empty top part of the dropper with the inert gas or vaseline or something like that?
-Put a few mg of EDTA?
Thanks!!!
I want to store for years.
G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,991
- Solutions
- 3
- Reaction score
- 3,378
- Points
- 113
- Deals
- 1
Keep receptacle tightly sealed. Store in cool (use -17-20 *C freezer), dry conditions in well sealed receptacles.
- Language
- 🇪🇸
- Joined
- Jul 11, 2023
- Messages
- 12
- Reaction score
- 5
- Points
- 3
How can I create an inert atmosphere? What devices do I need?
- Joined
- Aug 8, 2022
- Messages
- 674
- Reaction score
- 512
- Points
- 63
- Deals
- 5
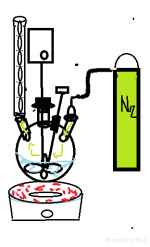
Here, I have illustrated a classic inert gas reaction setup. You can follow the flow of N2 to the condenser where it eventually gets pushed out, ensuring little to no oxygen can enter.The flow must be calibrated as such that nitrogen does not push out reaction products or solvents that we don't want pushed, to this a end a bubbler can be attached between the canister and flask to visualize the flow of gas.N2 is used in this way in official settings since it is readily available and cheap, but also light, so it needs to be flowing to not be replaced with athmospheric air.
We need only to use our imagination to apply this to our amateur setting.A gas that is inert and by contrast to N2 is much heavier is Argon, it is more expensive and is much rarer, but available.Argon can be used this way by only filling your reaction medium using a canister and optionally pulling a vacuum at the end of the condenser and refilling with argon.
We need only to use our imagination to apply this to our amateur setting.A gas that is inert and by contrast to N2 is much heavier is Argon, it is more expensive and is much rarer, but available.Argon can be used this way by only filling your reaction medium using a canister and optionally pulling a vacuum at the end of the condenser and refilling with argon.
Attachments
- Language
- 🇪🇸
- Joined
- Jul 11, 2023
- Messages
- 12
- Reaction score
- 5
- Points
- 3
G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,991
- Solutions
- 3
- Reaction score
- 3,378
- Points
- 113
- Deals
- 1
- Language
- 🇺🇸
- Joined
- Dec 21, 2022
- Messages
- 24
- Reaction score
- 5
- Points
- 3
Could lysergic acid methyl ester be used instead of ergotamine?
Would the basic condition hydrolize the methyl ester?
If this works the rest of the procedure would be the same.
Using methyl lysergate would make LSD synthesis really user friendly and cheap.
Any advice is appreciated
Would the basic condition hydrolize the methyl ester?
If this works the rest of the procedure would be the same.
Using methyl lysergate would make LSD synthesis really user friendly and cheap.
Any advice is appreciated
- Language
- 🇬🇧
- Joined
- Jan 25, 2022
- Messages
- 87
- Reaction score
- 73
- Points
- 18
Yes, you can hydrolize under the same conditions to cleave the additional groups
- Joined
- May 10, 2023
- Messages
- 17
- Reaction score
- 10
- Points
- 3
@G.Patton
Hi, can ergotamine tartrate be changed for "Dihydro Ergotamine Mesylate --> 6190-39-2"?
Adjusting molar ratios and such (obviously).
Perhaps we would obtain Lysergic acid diethylamide (LSD) mesylate? Or am I wrong?
Hi, can ergotamine tartrate be changed for "Dihydro Ergotamine Mesylate --> 6190-39-2"?
Adjusting molar ratios and such (obviously).
Perhaps we would obtain Lysergic acid diethylamide (LSD) mesylate? Or am I wrong?
G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 2,991
- Solutions
- 3
- Reaction score
- 3,378
- Points
- 113
- Deals
- 1
Yes, you can
You won't get LSD mesylate if just substitute ergotamine salt. You need to change acid in the last step. I'm not sure that you can get LSD mesylate in practice.
You won't get LSD mesylate if just substitute ergotamine salt. You need to change acid in the last step. I'm not sure that you can get LSD mesylate in practice.
- Language
- 🇬🇧
- Joined
- Jan 15, 2023
- Messages
- 609
- Reaction score
- 352
- Points
- 63
- Language
- 🇬🇧
- Joined
- Sep 26, 2023
- Messages
- 5
- Reaction score
- 1
- Points
- 3
Hello,
What solvent would you use for the purpose of recrystalising the D-Lysergic Acid Hydrate? Would ethyl acetate be suitable?
I also have a similar question for the recrystallisation of the lysergic acid diethylamide tartrate if there are any other solvents which the salt would have similar solubility to methanol?
Thank you very much
What solvent would you use for the purpose of recrystalising the D-Lysergic Acid Hydrate? Would ethyl acetate be suitable?
I also have a similar question for the recrystallisation of the lysergic acid diethylamide tartrate if there are any other solvents which the salt would have similar solubility to methanol?
Thank you very much
About Us
Our team brings together the best specialists from different fields.
We are ready to share our experience, discuss difficult issues and find new solutions.