- Joined
- May 23, 2023
- Messages
- 6
- Reaction score
- 4
- Points
- 3
Introduction:
A certain vendor online (that shall not be named) appears to be suggesting a "novel" route to phenethylamines.
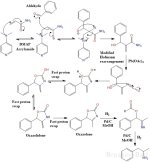
The reaction from aldehyde to amine occurs in 3 distinct steps:
1) A Baylis-Hillman reaction is first used to couple an aryl aldehyde with acrylamide using a tertiary amine base like
DMAP (4-dimethylaminopyridine) or DABCO/TEDA (triethylenediamine).
Acrylamide is an industrial chemical used to make polymers, it is also associated with burnt food.
2) A modified Hofmann rearrangement is then performed.
Unlike the "regular" Hofmann reaction, it is important to note that the final product is not an amine.
The intermediate isocyanate will not undergo hydrolysis by water to form the carbamic acid which is then decarboxylated to give the amine.
Instead, the benzyl alcohol formed in step 1 will undergo an intramolecular cyclization forming a 5 membered carbamate ring (which is both an ester and an amide).
A series of rapid proton swaps occur which then isomerize the unconjugated oxazolidone to the more stable conjugated oxazolone.
3) In the final step a palladium on carbon reduction is performed using 3 equivalents of hydrogen gas.
The first equivalent of hydrogen is used to break open the oxazolone ring, producing a formate ester and an imine which are then
reduced by the remaining 2 equivalents of hydrogen. The desired amine is formed along with formic acid as a byproduct.
It is possible that this reaction is already known within the clandestine scene (albeit a very well kept secret),
however, I dare to say the reaction is "novel" because I have never heard any discussions/documented reports of this reaction on any similar forums or in the literature.
The precise mechanism of the reaction is unknown although I gave my best attempt at elucidating the mechanism.
If you disagree with my mechanistic assumptions please explain your thoughts/criticisms with sound reasoning in the comments.
Your feedback is very much appreciated.
Literature evidence:
[1] Pedro Molina, Alberto Tarraga, in the book "Comprehensive Organic Functional Group Transformations", 1995, ISBN: 978-0-08-044705-6
"lead tetra-acetate in pyridine has been found to provide a mild procedure for effecting a rapid, high-yield, Hofmann-like rearrangement of β-hydroxy primary amides to 2-oxazolidinones, via the corresponding β-hydroxyisocyanate"
[2] Shinobu Hazama, S. Ichikawa, Fumihiro Yonebayashi, in the Japanese Journal of Forensic Science and Technology, 2008, DOI: 10.3408/jafst.13.67
"We developed a new and convenient method for the synthesis of levo-Methamphetamine using d-norephedrine (d-NE) as a starting material. d-NE was treated with 1,1-carbonyldiimidazole to produce the corresponding cyclic carbamate, and the product was treated with sodium hydride and iodomethane to form mono-N-methylated amine derivative, which was treated with palladium on activated carbon in hydrogen atmosphere for catalytic reduction. After the addition of aqueous hydrogen chloride (HCl), l-MA was obtained as its HCl salt (total yield 58%)"
Literature Mechanism/Reaction Scheme:

Proposed (Hypothetical) Mechanism/Reaction Scheme:

Questions that still need answering:
1) What are the exact reaction conditions (temp, conc, rxn time etc...) and reagents required for this reaction to work?
2) What are the work up conditions required to purify each intermediate?
3) I'm not sure how much testing and iterative development occurs on this forum.
Is anyone on the forum with a proper lab space willing to try this out and give feedback? I don't have the means to test this myself.
4) Is the reaction mechanistically sound? Does the reaction actually exist or did I just pull this out my a**?
5) Does a procedure already exist for this reaction? If so where can I find it?
6) Can the reaction scheme be modified to also include the synthesis of n-methylated derivates (similar to the example I showed in the literature).
7) Can alternate reagents to lead tetra-acetate be used to induce the cyclization step? I feel like
hypochlorite won't work because it would also oxidize the benzyl alcohol to the ketone/phenone.
8) Can other tertiary amines like triethylamine be used other than DMAP and DABCO?
9) Are there any noteworthy side reactions that would decrease the purity of the final product?
10) Will the reduction produce a racemic amine or will it preferentially favour one stereoisomer over the other?
This is my first post on this forum so I'm not sure what feedback to expect.
Thank you for reading.
A certain vendor online (that shall not be named) appears to be suggesting a "novel" route to phenethylamines.
The reaction from aldehyde to amine occurs in 3 distinct steps:
1) A Baylis-Hillman reaction is first used to couple an aryl aldehyde with acrylamide using a tertiary amine base like
DMAP (4-dimethylaminopyridine) or DABCO/TEDA (triethylenediamine).
Acrylamide is an industrial chemical used to make polymers, it is also associated with burnt food.
2) A modified Hofmann rearrangement is then performed.
Unlike the "regular" Hofmann reaction, it is important to note that the final product is not an amine.
The intermediate isocyanate will not undergo hydrolysis by water to form the carbamic acid which is then decarboxylated to give the amine.
Instead, the benzyl alcohol formed in step 1 will undergo an intramolecular cyclization forming a 5 membered carbamate ring (which is both an ester and an amide).
A series of rapid proton swaps occur which then isomerize the unconjugated oxazolidone to the more stable conjugated oxazolone.
3) In the final step a palladium on carbon reduction is performed using 3 equivalents of hydrogen gas.
The first equivalent of hydrogen is used to break open the oxazolone ring, producing a formate ester and an imine which are then
reduced by the remaining 2 equivalents of hydrogen. The desired amine is formed along with formic acid as a byproduct.
It is possible that this reaction is already known within the clandestine scene (albeit a very well kept secret),
however, I dare to say the reaction is "novel" because I have never heard any discussions/documented reports of this reaction on any similar forums or in the literature.
The precise mechanism of the reaction is unknown although I gave my best attempt at elucidating the mechanism.
If you disagree with my mechanistic assumptions please explain your thoughts/criticisms with sound reasoning in the comments.
Your feedback is very much appreciated.
Literature evidence:
[1] Pedro Molina, Alberto Tarraga, in the book "Comprehensive Organic Functional Group Transformations", 1995, ISBN: 978-0-08-044705-6
"lead tetra-acetate in pyridine has been found to provide a mild procedure for effecting a rapid, high-yield, Hofmann-like rearrangement of β-hydroxy primary amides to 2-oxazolidinones, via the corresponding β-hydroxyisocyanate"
[2] Shinobu Hazama, S. Ichikawa, Fumihiro Yonebayashi, in the Japanese Journal of Forensic Science and Technology, 2008, DOI: 10.3408/jafst.13.67
"We developed a new and convenient method for the synthesis of levo-Methamphetamine using d-norephedrine (d-NE) as a starting material. d-NE was treated with 1,1-carbonyldiimidazole to produce the corresponding cyclic carbamate, and the product was treated with sodium hydride and iodomethane to form mono-N-methylated amine derivative, which was treated with palladium on activated carbon in hydrogen atmosphere for catalytic reduction. After the addition of aqueous hydrogen chloride (HCl), l-MA was obtained as its HCl salt (total yield 58%)"
Literature Mechanism/Reaction Scheme:
Proposed (Hypothetical) Mechanism/Reaction Scheme:
Questions that still need answering:
1) What are the exact reaction conditions (temp, conc, rxn time etc...) and reagents required for this reaction to work?
2) What are the work up conditions required to purify each intermediate?
3) I'm not sure how much testing and iterative development occurs on this forum.
Is anyone on the forum with a proper lab space willing to try this out and give feedback? I don't have the means to test this myself.
4) Is the reaction mechanistically sound? Does the reaction actually exist or did I just pull this out my a**?
5) Does a procedure already exist for this reaction? If so where can I find it?
6) Can the reaction scheme be modified to also include the synthesis of n-methylated derivates (similar to the example I showed in the literature).
7) Can alternate reagents to lead tetra-acetate be used to induce the cyclization step? I feel like
hypochlorite won't work because it would also oxidize the benzyl alcohol to the ketone/phenone.
8) Can other tertiary amines like triethylamine be used other than DMAP and DABCO?
9) Are there any noteworthy side reactions that would decrease the purity of the final product?
10) Will the reduction produce a racemic amine or will it preferentially favour one stereoisomer over the other?
This is my first post on this forum so I'm not sure what feedback to expect.
Thank you for reading.
Attachments
Last edited: