- Language
- 🇬🇧
- Joined
- Sep 7, 2022
- Messages
- 39
- Reaction score
- 16
- Points
- 8
- Deals
- 9
Hi mate. I have a few questions about this part of your procedure
Do you leave the mixture at 112c until water boils away or just get to that temp and pour straight in the bucket?
In the bucket you have cold acetone again?
Do you not loose loads of product in the remaining water?
Thanks
Do you leave the mixture at 112c until water boils away or just get to that temp and pour straight in the bucket?
In the bucket you have cold acetone again?
Do you not loose loads of product in the remaining water?
Thanks
↑View previous replies…
- Joined
- Feb 7, 2023
- Messages
- 58
- Reaction score
- 21
- Points
- 8
there are so many methods that even people are confused... I used this method and it doesn't work... some say crystallization at room temperature with cold acetone... others say it's by heating
↑View previous replies…
- Joined
- Feb 14, 2023
- Messages
- 94
- Reaction score
- 45
- Points
- 28
- By bblanco
shulgin writes this:
"The actual form that the final salt takes depends upon the temperature and concentration at the moment of the initial crystallization. It can be anhydrous, or it can be any of several hydrated forms. Only the anhydrous form has a sharp mp; the published reports describe all possible one degree melting point values over the range from 148-153 ° C. The variously hydrated polymorphs have distinct infrared spectra, but have broad mps that depend on the rate of heating. "
"The actual form that the final salt takes depends upon the temperature and concentration at the moment of the initial crystallization. It can be anhydrous, or it can be any of several hydrated forms. Only the anhydrous form has a sharp mp; the published reports describe all possible one degree melting point values over the range from 148-153 ° C. The variously hydrated polymorphs have distinct infrared spectra, but have broad mps that depend on the rate of heating. "
- Language
- 🇬🇧
- Joined
- Jan 6, 2023
- Messages
- 279
- Reaction score
- 115
- Points
- 43
to be honest, I really like the method of crystallization of oversaturation solutions MDMA HCl + water, described by btcboss.
I did try it and it works perfectly if certain conditions applied. This shall be really calm place, without air flows and vibrations.
Once I did the stupidity - turned on the washing machine while crystallization was going on. As the result crystals grown not so nice and a kind of "small"
I did try it and it works perfectly if certain conditions applied. This shall be really calm place, without air flows and vibrations.
Once I did the stupidity - turned on the washing machine while crystallization was going on. As the result crystals grown not so nice and a kind of "small"
- Language
- 🇬🇧
- Joined
- Jan 6, 2023
- Messages
- 279
- Reaction score
- 115
- Points
- 43
check this picture, pls., it explains the importance of correct temperature pretty well.
if temperature of the solution grows, the saturation grows as well.
you can also read about Raoult's laws. briefly, is says: boiling temperature of the solution depends on the molar quantity of saturated salt. so, this temperature 108C suggested by @btcboss2022 probably reffers to the highest point of the "size" graph.
on the other side, the impurities become crystallization centers as well, so "dirty" solution will not bear you big crystals.
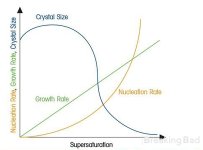
if temperature of the solution grows, the saturation grows as well.
you can also read about Raoult's laws. briefly, is says: boiling temperature of the solution depends on the molar quantity of saturated salt. so, this temperature 108C suggested by @btcboss2022 probably reffers to the highest point of the "size" graph.
on the other side, the impurities become crystallization centers as well, so "dirty" solution will not bear you big crystals.



