I'll put this here also:
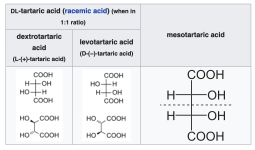
What really messed with my head in this description was the nomenclature concerning the tartaric acid. The text in the description uses the old nomenclature for the tartaric acid isomers, with lowercase "d" for dextro-tartaric acid. In modern texts and when you buy tartaric acid a capital "L" will be used for the same isomer: L-tartaric acid. So "d-tartaric acid" is the same thing as "L-tartaric acid".
To confuse things further, the other isomer, Levo-tartaric acid, is named "l-tartaric acid" in older texts, but "D-tartaric acid" in modern texts and in chemical companies.
Now,
D-tartaric acid (formerly
l-tartaric acid) is really expensive and difficult to buy. Often times it requires an End User Declaration to purchase in larger quantities.
L-tartaric acid (formerly
d-tartaric acid) is however both cheap and accessible. It's a natural acid derived from fruits and available to buy from winemaking stores online. This is the one you want.
it's also very usable to resolve (it's called
resolving when you separate enantiomers) methamphetamine, which is even more crucial since the L-isomer of meth is useless.
There's a pretty good article about resolving amphetamine isomers here, called "The Dutch Solution": (WARNING clearnet)
https://www.thevespiary.org/rhodium/Rhodium/chemistry/amphetamine.resolution.html
This is an excerpt from the text concerning the separation of meth isomers:
Resolution of racemic Methamphetamine
85 parts of racemic methamphetamine are introduced into a solution of 100 parts of d-tartaric acid in 1000 parts of methyl alcohol. After protracted standing about 100 parts of the precipitated salt are aspirated off and extracted with hot ethyl alcohol. Since the d-tartrate of dextrorotary methamphetamine is readily soluble in both methyl and ethyl alcohol whereas the d-tartrate of levorotary methamphetamine is sparingly soluble both in methyl alcohol and hot ethyl alcohol an extremely simple separation of the d-tartrates of the optical antipodes of the base is effected.
Reference: British Patent 508,757
So basically you dissolve meth or amph freebase in near-boiling methanol with L-tartaric acid in. Once the solution cools, crystals of l-amph/l-meth bound to L-tartaric acid will begin to crash out. You just filter these out and discard or save them.
I would then basify the solution with NaOH and evaporate off much off the alcohol, and add water to get a good separation of the freebase layer, extract it in a sep funnel and then bubble HCl gas through it if it's d-meth freebase or add sulphuric acid if it's d-amph freebase to get nice d-crystals.